Schematic: Cooling the Hottest Surfaces
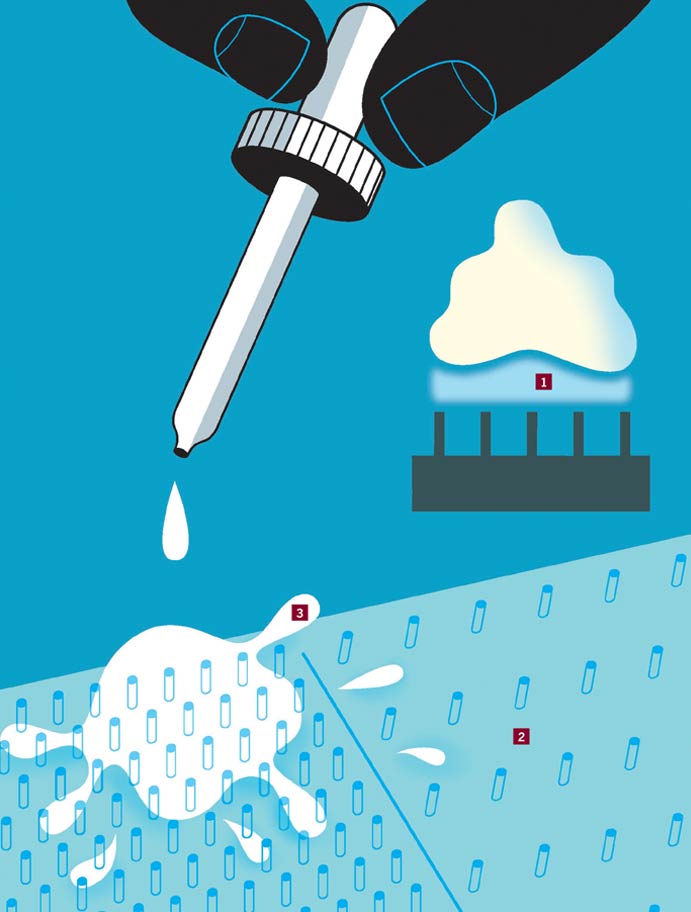
As it approaches an object that is much hotter than its boiling point, a liquid forms a vapor that prevents its droplets from making contact with the object's surface. This repulsive force—the Leidenfrost effect—causes droplets to boil off more slowly than they would on a cooler surface.
Engineers at the City University of Hong Kong, the Hong Kong University of Science and Technology, and Lehigh have learned to control the movement of liquid droplets and cool surfaces more efficiently. The discovery could improve technologies involving microfluidics, heat transfer, heat exchange and similar phenomena.
1. Vapor: Inside the vapor caused by the Leidenfrost effect, droplets of liquid hover over a surface, failing to achieve efficient heat transfer and desired surface cooling.
2. Patterns: To guide the liquid droplets and cool a surface more efficiently, researchers lithographically patterned a surface with micropillars and arranged them in zones that vary according to pillar density and contact angle.
3. Kinetics: These microscale features convert the excess surface tension of droplets into a kinetic energy that propels them to hot spots on the surface. The kinetic energy, Lehigh's Manoj Chaudhury, City University's Zuankai Wang and the other researchers wrote in Nature Physics, causes droplets "to dislodge from the surface, take flight into the air [and] eventually get deposited in the contact-boiling region."
Posted on:

